Business Business Area
Clean Room System
Semiconductor / General Cleanroom
Cleanroom Structure
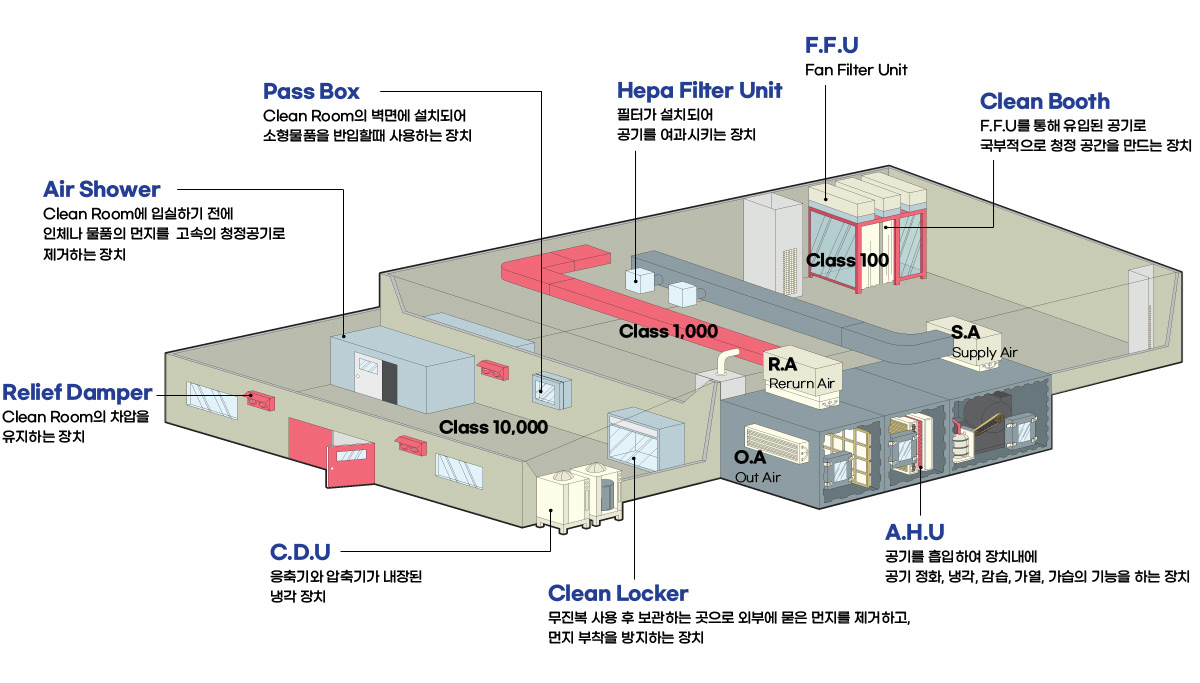
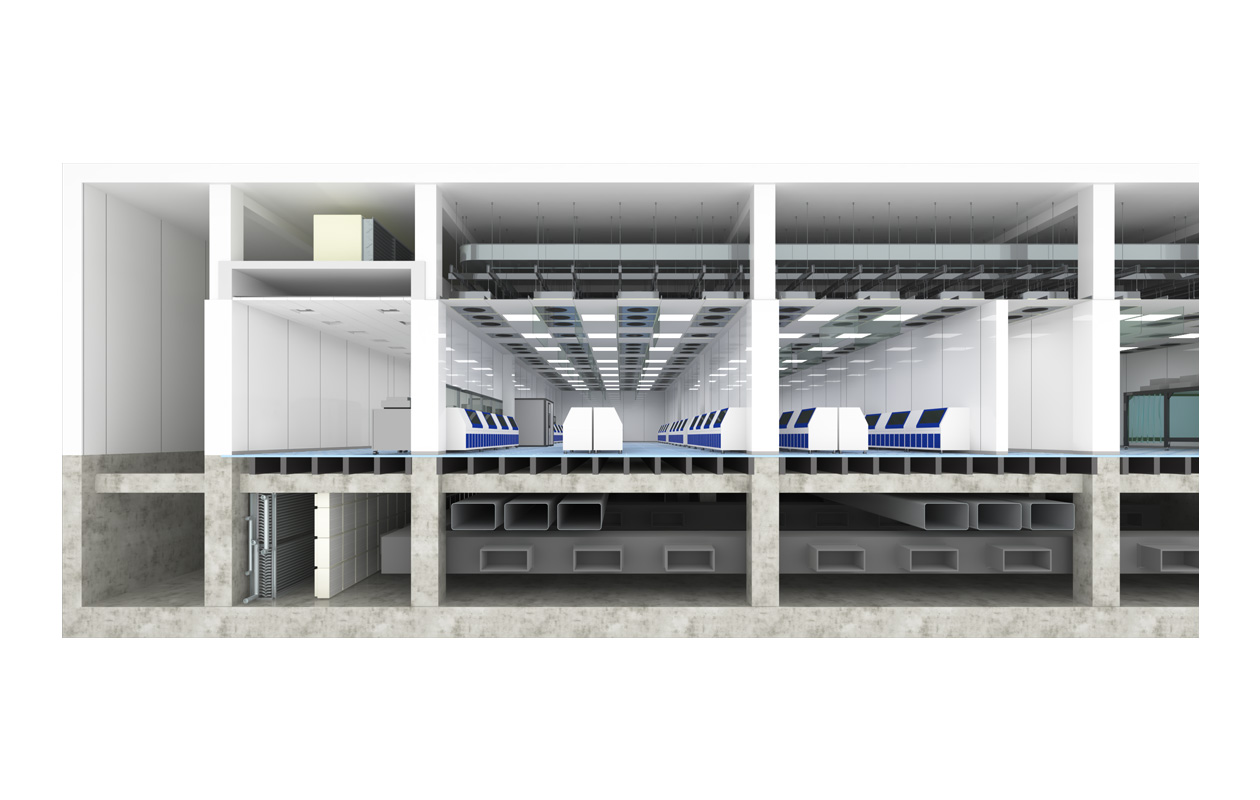



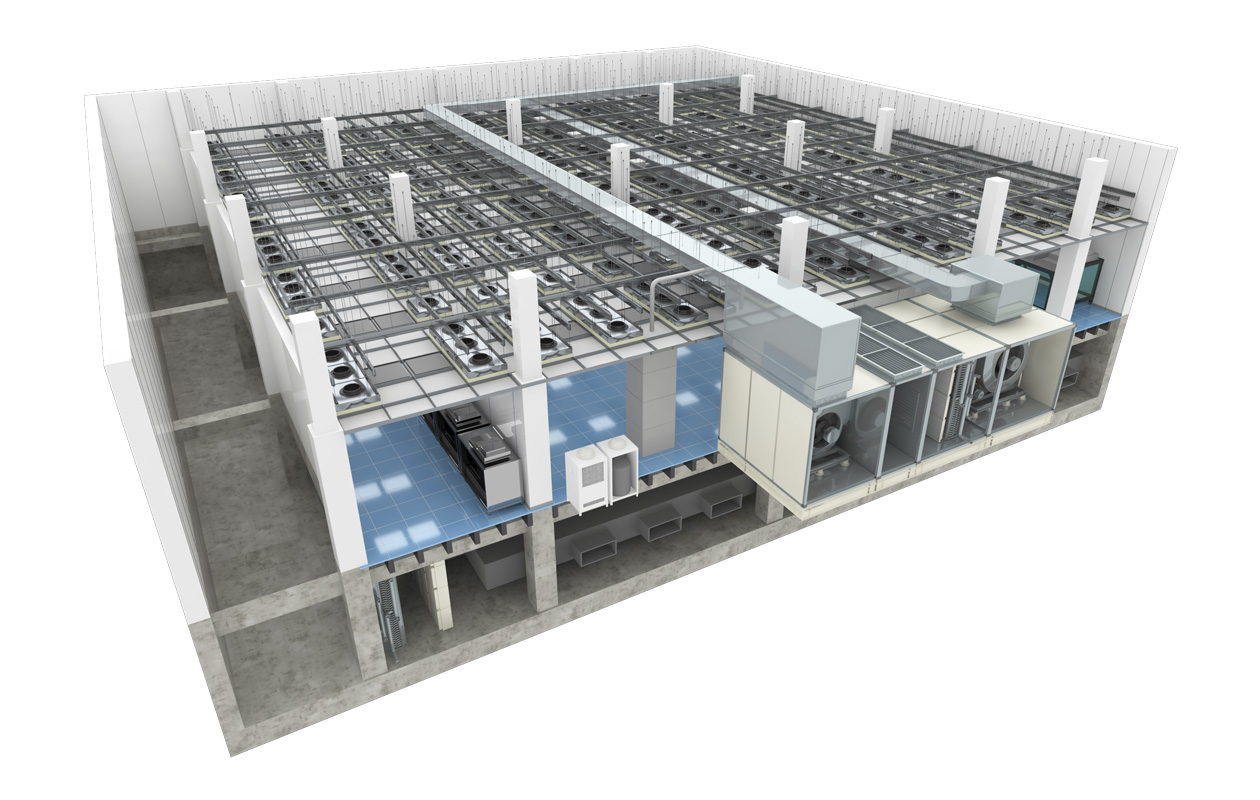
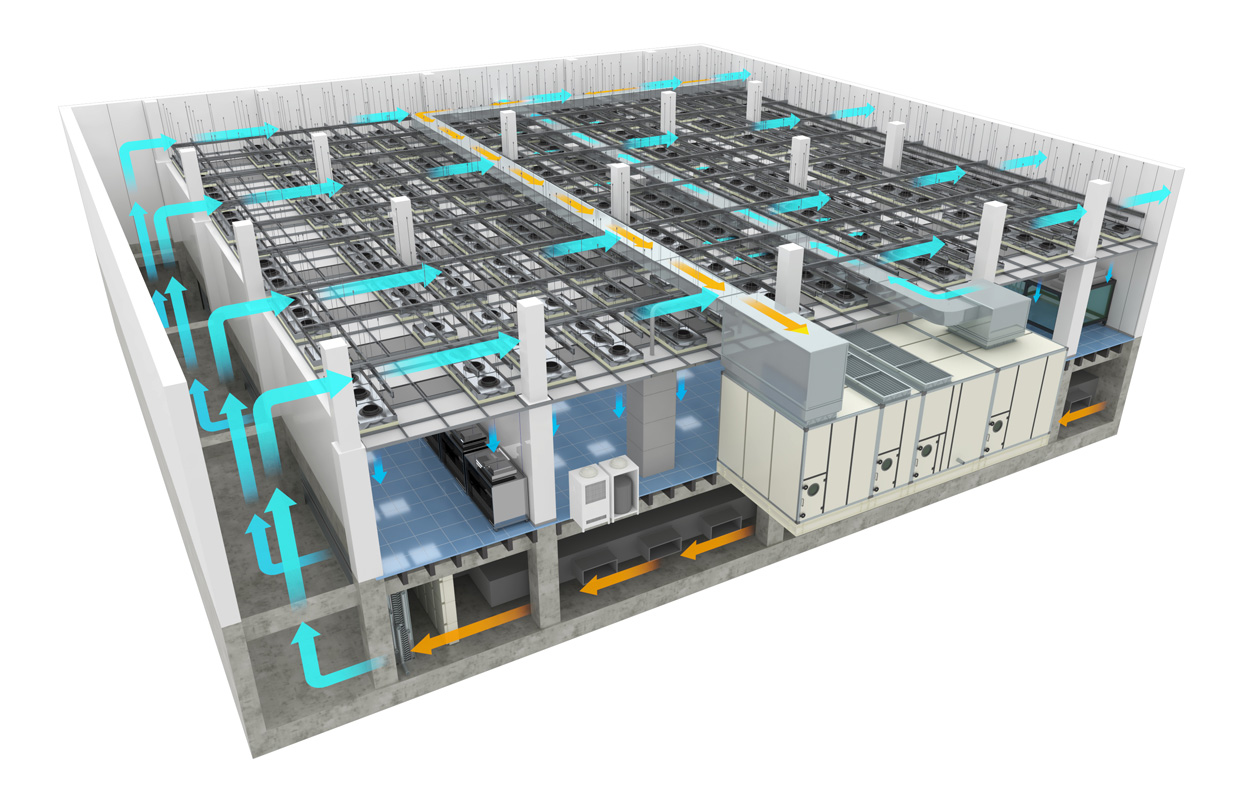
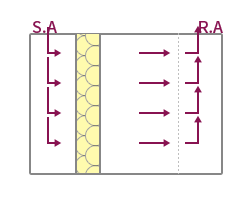
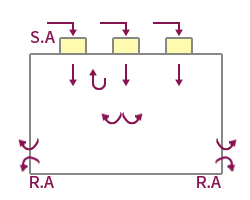
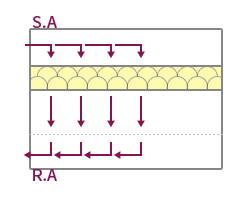
Classification in the Cleanroom according to the airflow method
| Item | Vertical Laminar Flow | Horizontal Laminar Flow | Turbulent Flow | Tunnel Method (CTM) |
| Cleanliness | Class 1~100 | Class 100 | Class 1,000~100,000 | Class 1~100 |
| Operating Cost | High | Medium | Low | Medium |
| Layout Change | Easy | Difficult | Easy | Difficult |
| Features | The method suitable for maintaining high cleanliness forms a vertical laminar flow, and the indoor wind speed is formed at 0.25~0.5m/s. | Applied to maintain high cleanliness by forming a horizontal laminar flow, but the cleanliness of the upper and lower layers may vary depending on the working conditions. | Applied to medium-grade cleanrooms by forming a vertical turbulent flow, forming an uneven and irregular airflow distribution. Ventilation frequency 20~80 times applied. | It is possible to form a unit type by applying a local vertical laminar flow method, and it is easy to maintain local high cleanliness. |
| Expandability | Difficult | Difficult | Somewhat Difficult | Expandable for each line |
| Applicable Equipment | FFU System, A/F System, Dry Coil System, OAC System | AHU System, Air Filter System | HFU System, BFU System, AHU System | CTM System, A/F System |
| Image | 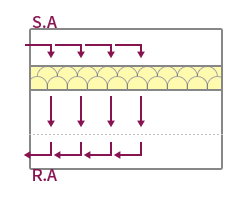 |
 |
 |
 |
Cleanroom Specifications: Cleanliness Standard
| CLASS | PARTICLE (EA/μm) | ||||
| 0.1μm | 0.2μm | 0.3μm | 0.4μm | 0.5μm | |
| 1 | 35 | 7.5 | 3 | 1 | - |
| 10 | 350 | 75 | 30 | 10 | - |
| 100 | - | 750 | 300 | 100 | - |
| 1,000 | - | - | - | 1,000 | 7 |
| 10,000 | - | - | - | 10,000 | 70 |
| 100,000 | - | - | - | 100,000 | 700 |
Cleanroom (ICR) Construction Sites






-
- GMP
GMP (Good Manufacturing Practice) is a set of management standards necessary for all processes from the purchase of raw materials to manufacturing and shipping in a factory to produce excellent pharmaceuticals. It requires a cleanroom facility that meets these standards and must obtain GMP facility certification from the Food Promotion Agency. It can also be applied to cosmetic manufacturing facilities and health functional foods in addition to pharmaceutical factories.
-
- GLP
GLP (Good Laboratory Practice) is a standard for transparently managing all information on toxicity tests conducted to ensure the safety of pharmaceuticals. A cleanroom facility (germ-free animals, SPF breeding rooms) that meets these management standards must be established.
-
- HACCP
HACCP (Hazard Analysis and Critical Control Point) is a scientific hygiene management system to ensure food safety through autonomous, systematic, and efficient management by identifying hazard factors that may occur at each stage from food raw materials to manufacturing, processing, preservation, distribution, cooking stages, and before final consumers consume, and determining critical control points to manage these factors intensively.






Cleanroom (GMP) Construction Sites














